Fundamentals of Neuroscience
Published on August 18, 2021by Le Mai Tan Dat
Hello, it's me again. Curious about what's new? I have completed the first part of my course "Fundamentals of Neuroscience," taught by Professor David Cox of Harvard University. This is a MOOC, and you can easily find it online. I plan to share what I've learned and my experiences enrolling in this course. However, covering everything in a single post would be overwhelming, so I will divide it into four parts. Please note that my understanding may not be entirely accurate since I am only a high school student, so I apologize for any mistakes. I would greatly appreciate hearing your thoughts. I will use the present tense for convenience.
Let's get straight to the point. Part 1 covers the electrical properties of neurons. This section is divided into four smaller parts, which I'll refer to as lessons. I will briefly outline the content of each lesson to provide an idea of what to expect in this course, in case any of you are considering taking it.
- —
Lesson 1: The Resting Potential
- —
Lessons 2: Passive Membrane Properties
- —
Lesson 3: The Action Potential
- —
Lesson 4: Action Potential Propagation
In lesson 1, we gain an insight into the basis of electricity in neurons. Some of you may have already known that neurons communicate with each other by sending out electrical signals, and there are elaborate mechanisms behind this seemingly simple idea. When at rest, neurons also have electricity in itself, this is called the resting potential. Take note that when measuring the membrane potential, neuroscientists always use the outside of the cell as "ground" or reference point, so the value of the resting potential is around - 75mV. Then we are introduced to two forces that cause the resting potential: diffusion and electrostatic forces. There are even illustrations and animations to aid us in understanding the activities of these two forces. The process is quite hard to explain in limited words so you can search for it online. Then we come to study the Nernst potential equation, which allows us to calculate an individual ion's potential at equilibrium. Again, take note that equilibrium does not mean that there is no movement of ions, it's just the movement of ions in and out of the cell are equal. The other formula is the GHK equation, which is kinda similar to the Nernst equation but allows us to calculate resting potential of various ions in the neurons, and is named after three prominent scientists in field: David Goldman, Alan Hodgkin and Bernard Katz. Hmm, but how can neurons allow for the passing of one particular kind of ions to create the resting potential? It is the work of ion filters. Nature has a lot to surprise us, and one of which is ion filters. For ions with opposite charges like Cloride and Sodium, it would be an easy work (same charges repel, opposite charges attract) but how about Sodium and Potassium, they are both positive and their sizes are incredible small, so how can we filter them? In fact, in aquatic solution, there are a myriad of water molecules roaming in the solution, when an ion comes into contact with water, these water molecules would be attracted to that ion (since water molecule is polarised, the positive end of it will go away from the Sodium ion (as an example) while the negative end of it surrounds that ion). This happen in a way that form a solvation shell around the given ion, and each ion has its unique solvation shell. Thanks to this, ion channels (made up of protein) would replicate the shape of the solvation shell and therefore, while one ion can flow through with ease, the others wouldn't. Fascinating, isn't it? We also learn about the active Na+/K+ bumps, which is already covered in Biology classes at school. After each lesson, we are taken to a field trip and for lesson 1, we are allowed to join the virtual visit of the Museum of Science to understand more about interaction between charges and we attend a class about human brain dissection. Although the content of the course is quite academic, it is in fact enjoyable since the layout is user-friendly and the explanations are straightforward and comprehensible. I don't wanna spoil the fun so I skipped through all of the academic features, if you want to know more, just contact, I'm willing to guide you through this.

Above is the structure of a neuron, as you can see, a neuron is mainly made up of a cell body, pendrites and an axon. The outer surface of the neuron is the bilayers of lipids. In the axon, we can see the myelin sheath, the is a wonder of nature and I will go into details later.
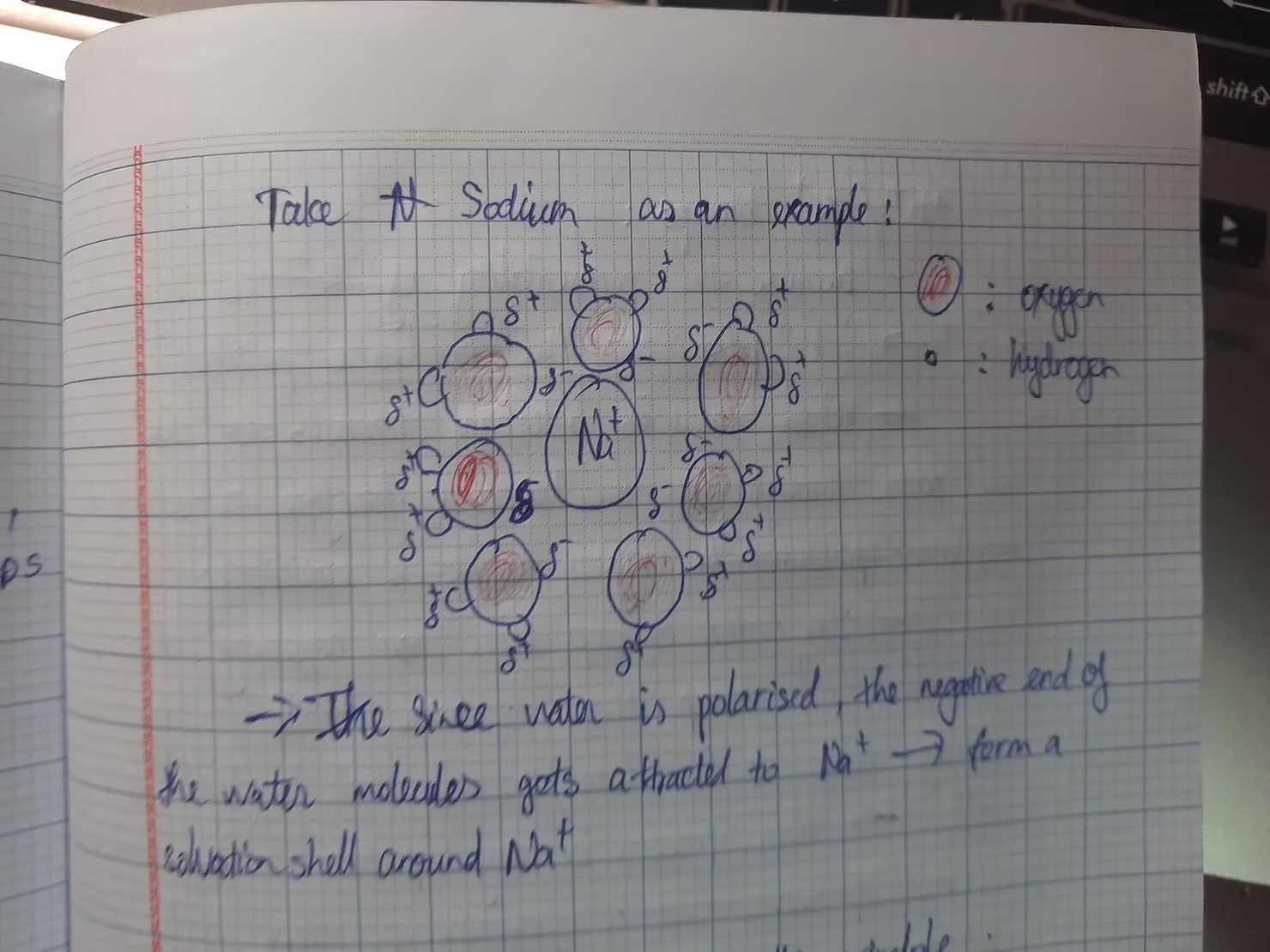
Above is the solvation shell of Sodium, it looks kinda like this, this is just a simple drawing for you to understand with ease.
In lesson 2, we explore Passive Membrane Properties. These properties are quite similar to concepts in Physics, so with a solid background in physics, you should find it easy to grasp the ideas of membrane resistance, axial resistance, and membrane capacitance. These concepts adhere to Ohm's law, implying that reducing resistance facilitates the flow of current along an axon. We also discuss the length constant and time constant, which represent the distance a current can travel before diminishing to about 37% of its initial value and the time required for a membrane segment to charge up to 63% of its final value, respectively. It may seem complex, but the course provides clear explanations. Simply put, the length constant is the distance a current travels before decreasing to a certain value, and the time constant is the time needed to charge the membrane potential to a specific value. Since the membrane is composed of lipid bilayers, it acts as a capacitor, buffering changes in voltage across the membrane. This means that when an electrical signal is applied to a neuron, the response is gradual rather than instantaneous. There are various ways to modify the resistance or capacitance of a neuron, and nature has ingeniously adapted these mechanisms, which I will elaborate on later.
Regarding the field trip, we have the opportunity to gain insights into the case of Phineas Gage. You can find information about this online, and our professor is taking us to the Warren Anatomical Museum to view the exhibits. I learned about his case previously in an extra class with Mrs. Alix Alicia. She taught us about his incident, and it's remarkable how the brain could endure such severe damage. Naturally, Gage experienced personality changes, transforming from a violent individual to an artistic one, to the point where even his wife struggled to recognize him. Despite his extraordinary artistic talents, Gage's works do not express happiness or pleasure but rather convey a sense of distress, haunting viewers. It seems that genius often comes with suffering. It's unfortunate that humans cannot fully enjoy everything they possess, as everything has its cost. Nevertheless, I am hopeful for the future of humanity, where brain trauma might not be synonymous with death and suffering, although this is unlikely. Gage's case remains a mystery to be unraveled, and I invite you to join me on this journey of discovery.
In lesson 3, we delve into the concept of The Action Potential. As we discussed in the first part, neurons communicate by transmitting electrical signals. These changes in membrane potential must occur rapidly, as we typically do not spend more than a minute to perform simple actions like picking up a pen. When ion channels open, Na+ ions enter the cell, bringing the membrane potential closer to its Nernst potential (which is relatively high and positive), while K+ ions exit the cell, moving the membrane potential toward its own value (slightly lower than the resting potential). Without further intervention, the membrane potential would remain at its resting value, preventing any electrical signaling in neurons. This is where voltage-gated channels become essential. There are two types of voltage-gated channels: Na+ and K+. These channels are particularly sensitive to changes in membrane potential, and their conductance varies accordingly. Sodium voltage-gated channels open when the membrane potential becomes more positive, allowing Na+ to flow through and further increase the membrane potential. Does this create a continuous loop? Fortunately, sodium voltage-gated channels possess a unique mechanism: a ball at the end of the pore closes the channel when the membrane potential reaches a certain level, preventing Na+ from passing through. This is known as the inactivation stage. The potassium voltage-gated channels then reset the membrane potential by opening when it becomes more positive, permitting the passage of K+. However, this process is probabilistic; while voltage-gated Na+ channels generally open at more positive potentials, they can also do so at very negative values, albeit less frequently. Both channels open at more positive membrane potentials—why don't they negate each other? This is due to channel kinetics. The inactivation of sodium voltage-gated channels and the opening of potassium voltage-gated channels occur more slowly than the opening of sodium voltage-gated channels. Channel kinetics also contribute to the refractory period: absolute, when sodium voltage-gated channels are inactivated (preventing immediate stimulation of another action potential), and relative, when both sodium and potassium voltage-gated channels are open (making it difficult to stimulate another action potential).
Now, let's discuss the field trip content. We will visit an aquarium, where professors and the aquarium's caretaker will provide fascinating insights into neurotoxins—chemicals that influence neuronal activity—specifically tetrodotoxin (TTX). This toxin, commonly found in puffer fish, binds to sodium voltage-gated channels, which is why consuming this fish can lead to numbness. We will also explore the electroreceptors found in some fish.
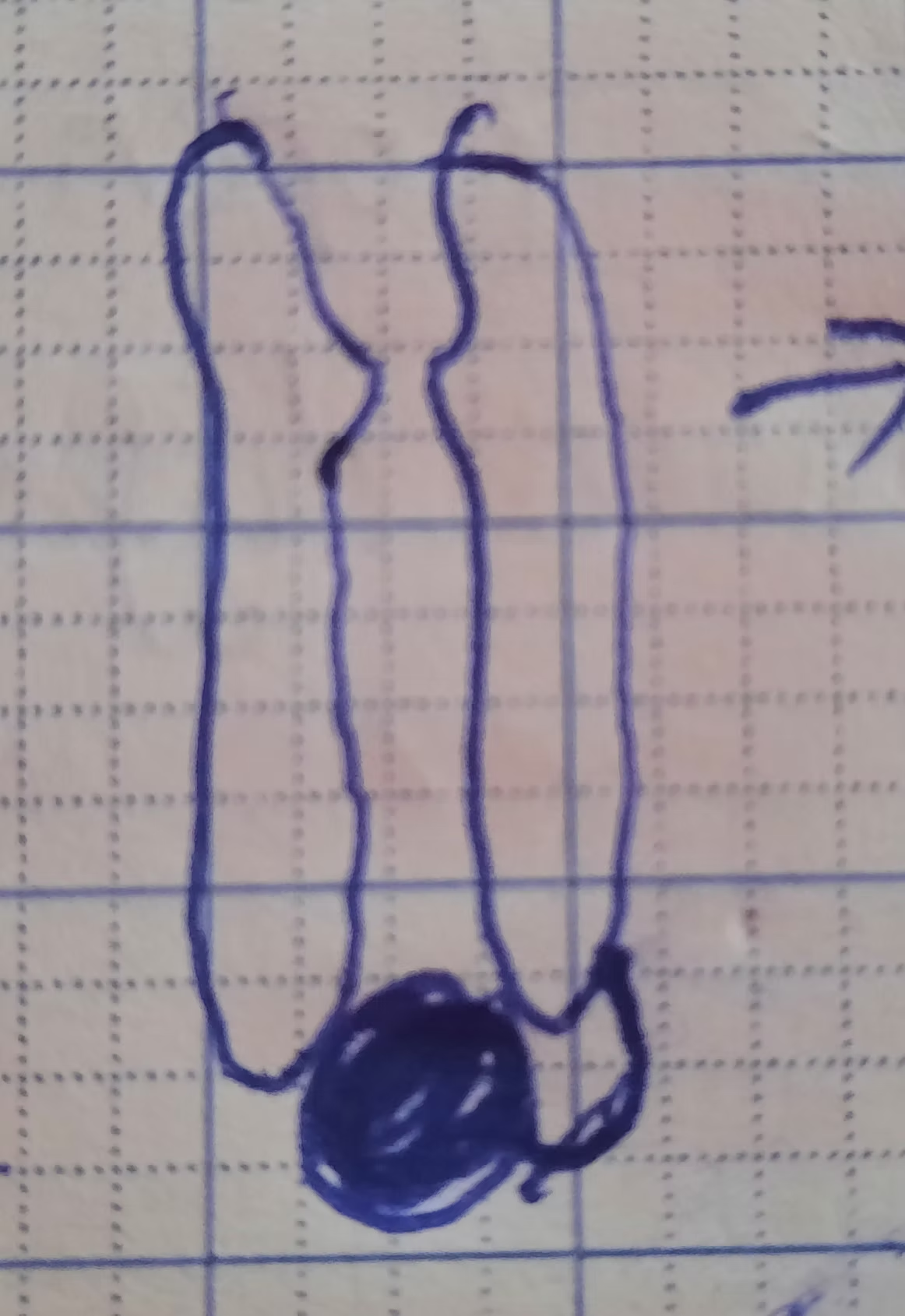
Above is the shape of a Sodium voltage-gated channel at inactivation stage (you can clearly see the ball at the end of it).
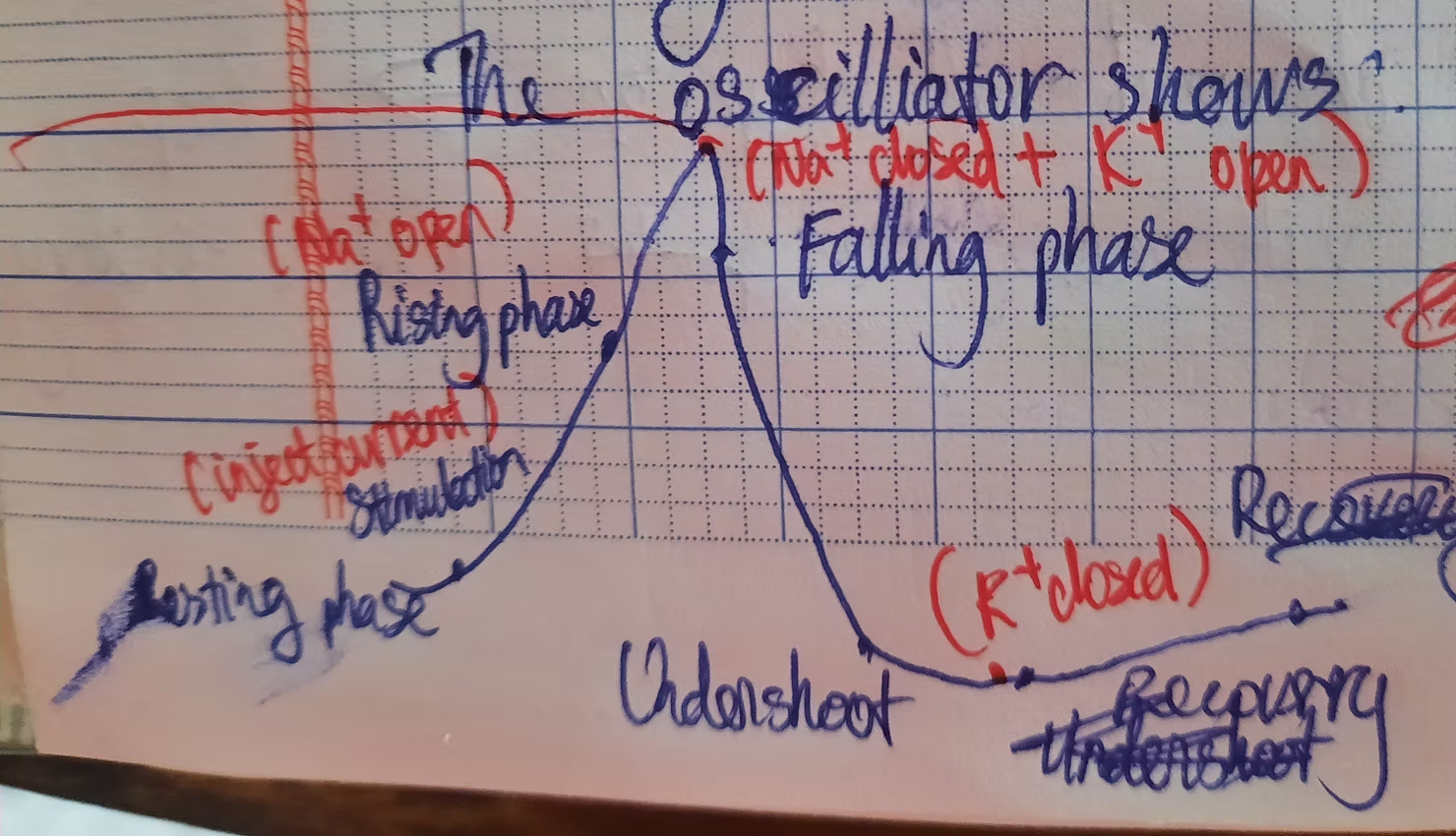
Above is the shape of an action potential with corresponding stages.
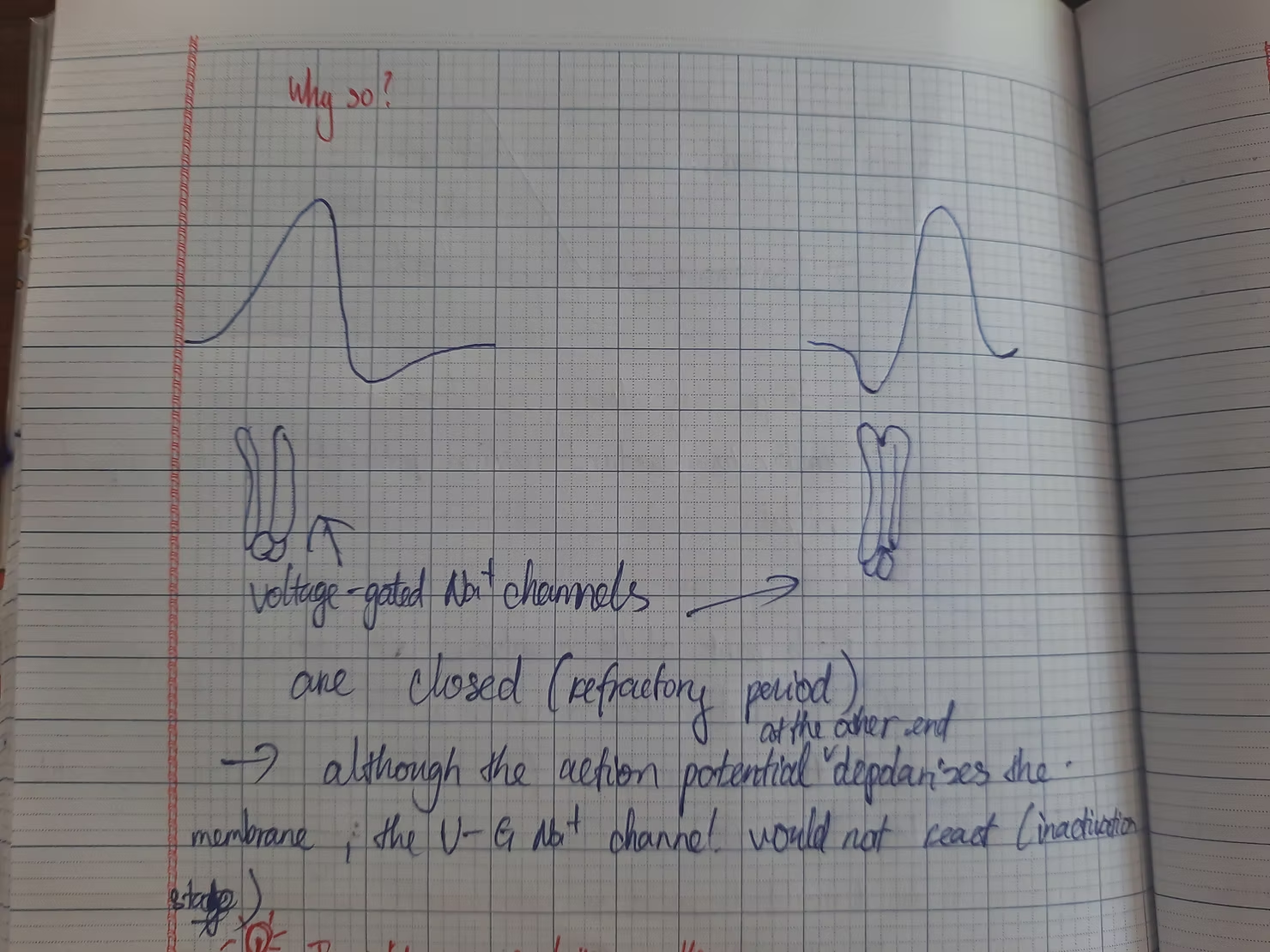
Let us come to the last lesson of the series. A wire transmits electricity based on the high conductivity of copper. However, the cytoplasm of neurons is significantly less conductive than copper, making it challenging for neurons to transmit signals using this method. Consequently, we have two options: increase the amplitude of the current or regenerate it repeatedly. While the first option may seem simpler, it risks damaging the neuron's membrane. Therefore, nature opts for the latter. Instead of moving current down an axon, neurons propagate a state of depolarization. Similar to a wave, where the propagation of up-and-down movements results in wave motion, in neurons, an action potential in one membrane patch depolarizes the adjacent region, triggering a chain reaction (domino effect). The voltage versus distance curve resembles the standard action potential shape but is flipped due to the wave effect (each membrane segment experiences an action potential continuously). To facilitate understanding, HarvardX offers interactive lessons that greatly aid in grasping these abstract concepts. Let's set this aside for now and explore some scenarios. First, if we stimulate an action potential in the middle of an axon, it will propagate to both ends of the neuron, as neurons lack directional filters. What if we stimulate an action potential at each end of the neuron? They would collide and cease due to the inactivation phase of the voltage-gated Sodium channels (or absolute refractory period). Below is an illustration for clarity. Researchers utilize this phenomenon to determine where a neuron's axon projects. To expedite action potential propagation, we can either use a giant axon, allowing for high-speed transmission but consuming substantial space, or employ myelinated axons. This is where it becomes intriguing. Glial cells (neuron support cells) create the myelin sheath that wraps around an axon. When an axon is myelinated, the membrane's thickness increases, reducing its capacitance and accelerating propagation speed. But doesn't myelination increase electrical resistance? Indeed, wrapping myelin around an axon reduces ion channels (these channels are covered), preventing current leakage and enabling longer-distance travel. So, is it best to myelinate the entire axon? Contrary to that notion, while current travels farther, it eventually dissipates. This is where the nodes of Ranvier come into play (gaps between myelinated axon segments). The nodes allow current to be reboosted, thanks to the ion channels present, ensuring high-speed action potential propagation. If the myelin sheath is damaged, current leaks through the membrane, dissipating before reaching a node of Ranvier. This leads to difficulties for patients with demyelinating diseases, often resulting in paralysis. This is indeed unfortunate. Scientists are tirelessly working to eliminate these diseases, and we should support their efforts.
The field trip includes a fascinating sheep brain dissection. However, I will not delve into this field trip now. Enroll in the course to experience it firsthand.
Take care and have a pleasant day. Thank you for your support.

Above is the picture of myelin sheath and nodes of Ranvier.
Above is the reason why two action potential at each end collide and disappear.
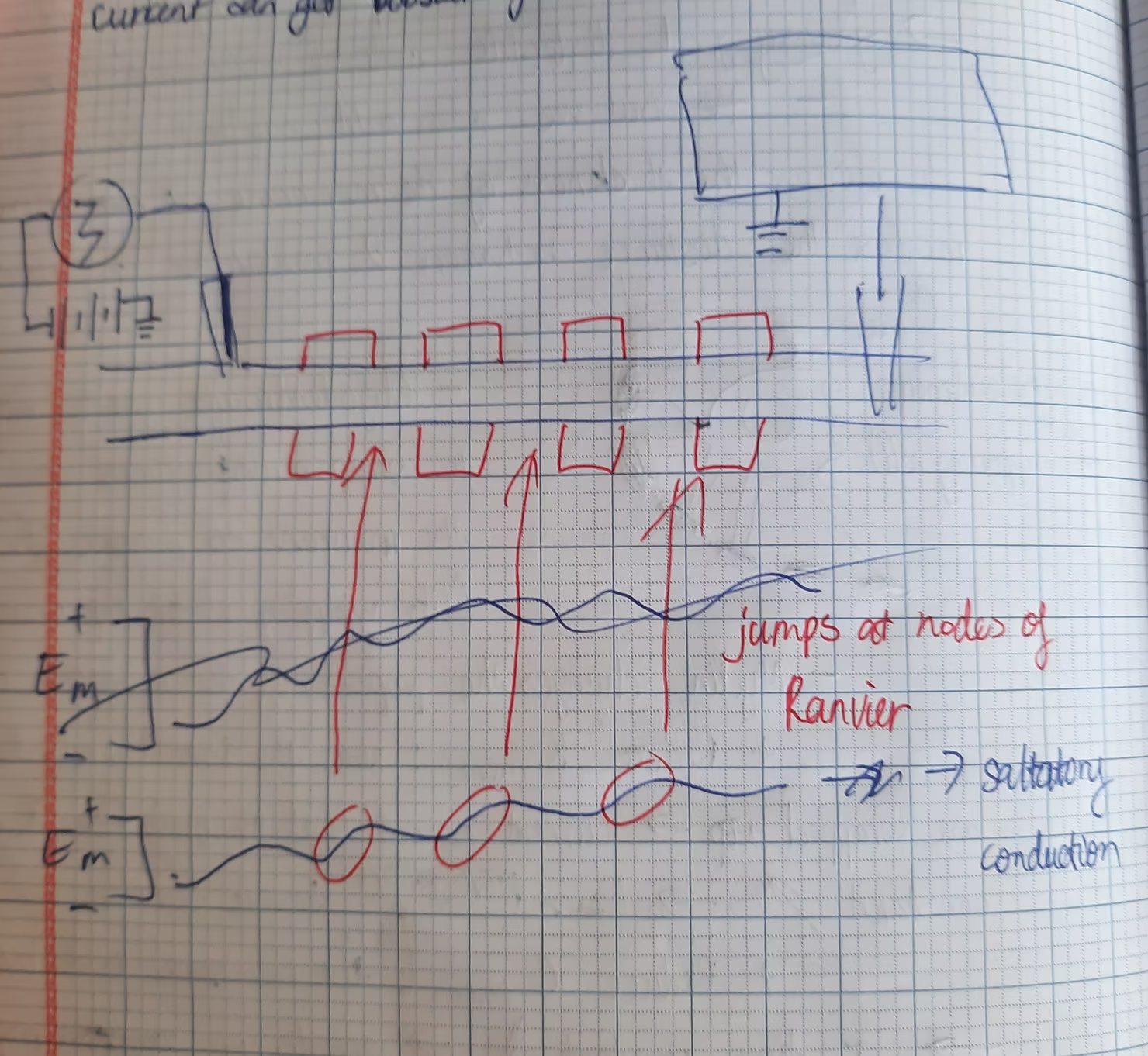
Above is the shape of the membrane potential when moving along an axon with myelin sheath.
August 2021, Vietnam.
____________________
Courses list:
David Cox. (n.d.). HarvardX: Fundamentals of Neuroscience, Part 1: The Electrical Properties of the Neuron [MOOC]. Edx. https://www.edx.org/learn/neuroscience/harvard-university-fundamentals-of-neuroscience-part-1-the-electrical-properties-of-the-neuron.
David Cox. (n.d.). HarvardX: Fundamentals of Neuroscience, Part 2: Neurons and Networks [MOOC]. EdX. https://www.edx.org/learn/neuroscience/harvard-university-fundamentals-of-neuroscience-part-2-neurons-and-networks.